Talk:Rankine cycle
First thing to show is p-v diagram.
Why is the word steam in the intro. — Preceding unsigned comment added by 128.118.7.237 (talk) 22:05, 22 November 2020 (UTC)
| This It is of interest to the following WikiProjects: | ||||||||||||||||||
‹See TfM›
| ||||||||||||||||||
Work: W=VdP vs W=-PdV
[edit]Could someone explain why in Process 3–4 the work is assumed to be equal to the change of enthalpy, rather than to the change of internal energy? For an adiabatic transformation, the work is equal to the change of internal energy (https://en.wikipedia.org/wiki/Internal_energy) dW=-PdV. For the work to be equal to VdP, the potential PV would have to be conserved ( d(PV)=0 ), which is true, for an ideal gas, only if the transformation is isothermal. Percht (talk) 21:06, 27 January 2021 (UTC)
Untitled
[edit]why is there no PV diagram? — Preceding unsigned comment added by 86.26.165.15 (talk) 12:53, 9 March 2015 (UTC)
If anyone can get the image fit more nicely on the page, feel free. I messed with it for quite a while to get it to fit at all.
I think the Equations section could use some more clarification. Varable definitions, dot represents time derivative (I assume?).--Cmprince 21:58, 10 Dec 2004 (UTC)
- Point taken. Adding variable definitions. --ABQCat 22:01, 10 Dec 2004 (UTC)
- Cool. I haven't had to do any thermodynamics in years, so I'd have had to dig out a book (horrors!) :) --Cmprince 22:36, 10 Dec 2004 (UTC)
- Yeah. I noticed that there was no article on the Rankine cycle after I finished with my thermodynamics course last semester, and decided (perhaps as a form of therapy to deal with the horrid subject matter?) to regurgitate what I had managed to learn and what I found useful into this article. Hopefully it'll help other people who are just as clueless as I was at points. --ABQCat 22:40, 10 Dec 2004 (UTC)
Test in introduction is wrong
[edit]It is stated in the introduction that: "This cycle generates about 90% of all electric power used throughout the world". The reference is from 2000 and is only for USA. Looking at the Key World Energy Statistics 2011 published by the International Energy Agency one sees at page 24 that hydro is 16.2% in 2009. We need to also take into account Brayton cycle and combined cycles. So I am fairly new here in the editing process... do I just delete the statement. Volusteinn (talk) 18:20, 5 March 2012 (UTC)
- I agree, I looked at some IEA reports on electricity production by fuel and I counted coal, nuclear, biomass and solar thermal. Natural gas and petrol(diesel) would be used in Brayton and Diesel cycles respectively. By my crude figure, I think we can safely say that at least 55% of world wide electricity production in 2009 was by rankine cycle steam, if all fuels above were solely used for it. Unfortunately, I have searched for world electricity production by generation method to no avail and this was the closest I got. It also means it is not a verifiable source.150.203.45.42 (talk) 03:55, 4 April 2012 (UTC)
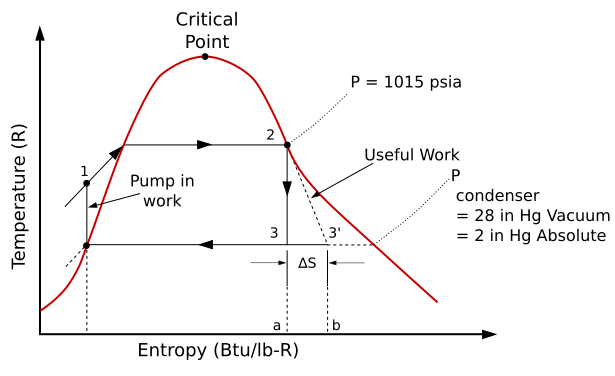
Diagram doesn't match text
[edit]The text (correctly) describes an ideal Rankine cycle as "heated at constant pressure by an external heat source to become a superheated vapor", but the diagram shows the vapor heated to the critical vapor point, not superheated.
Anyone have a better diagram handy? -- Kaszeta 15:30, 9 August 2005 (UTC)
- As I recall, I picked this image because I had extracted it from a government publication. Not really feeling like creating my own image (which I may spend some time on here if a search is unproductive), I used it. Not sure if the text describing the superheating was written by me or a later contributor, but you're definitely correct about it not matching the image.
- As a side note, and in case I'm perhaps being dense (despite having checked a few dusty textbooks), the equations were re-numbered recently such that heat processes were swapped for work processes. I'm quite certain I'm correct, but would value feedback here if I'm not. --ABQCat 08:17, 19 December 2005 (UTC)
- Same publication has a somewhat appropriate version of the image showing superheating. I'll take a closer look, but if it seems appropriate I'll upload it and replace the current image. --ABQCat 01:13, 20 December 2005 (UTC)
The equations do not match the diagram. The subscripts on the enthalpies are incorrect, indicating that Qin and Qout are on the left and right sides of the figure, which is incorrect.
I've changed the equations, and I've also edited the image for clarity. --Dric dolphin 01:52, 5 September 2006 (UTC)
I have been taught by two professors, one at UCLA and one at UC Davis, that the turbine compression cannot occur within the vapor dome, otherwise you will destroy your turbine. Maybe a different diagram should be used? — Preceding unsigned comment added by Clinsoccer (talk • contribs) 06:18, 8 March 2018 (UTC)
This point is discussed under 'Real Rankine cycle (non-ideal)'. But I agree that the first T-s diagram of a typical Rankine cycle operating between pressures of 0.06 bar and 50 bar needs to be modified as it is incorrect in showing the path between 2 and 3 as following the vapor dome (which would be inconsistent with a constant pressure process). One can see this by comparing the path 2-3 for this diagram and the subsequent one, which I think is correct.Bruckner130564 (talk) 09:53, 26 August 2021 (UTC)
Efficiency
[edit]Some typical and maximum efficiency levels would be useful. Tobyw 12:14, 8 March 2006 (UTC)
- "However, the thermal efficiency of actual large steam power stations and large modern gas turbine stations are similar." Large hardcoal fired power stations (new) have an eta_el = 46%, whereas the best gas turbine is around 40% - however CCGT go beyond >60%. So this sentence should be amended or stated more precisely. --Gunnar (talk) 06:55, 26 July 2018 (UTC)
Missing/empty link
[edit]There's an empty link, [[]], in the Regenerative Rankine cycle subsection near the bottom. I would have fixed this myself, but I don't know what it's supposed to say.
- It was empty as a result of vandalism by 213.181.226.27 on 26th October 2005. I fixed it.
New graph.
[edit]I've uploaded a new vector version of the graph to the wiki commons.
 Although I applied to make it almost pixel-perfect, I would like someone more confortable with this page to move on the article.
As you're reading this, I will have marked the old image as superceded.
Although I applied to make it almost pixel-perfect, I would like someone more confortable with this page to move on the article.
As you're reading this, I will have marked the old image as superceded.
MaxDZ8 talk 16:04, 24 May 2007 (UTC)
Rewrite (and a question)
[edit]I've reworded the processes section with a new diagram, Data was plotted from IAPWS IF-97 in SI units. I'll be changing other parts of the article very soon too, equations need tidying up a little and perhaps some more info about where this cycle is used and why it is used above others. Also super heating among other things needs covering. I've also made another diagram of a schematic that I'll try to include, Ts diagrams don't mean all that much to non-Engineering/Physics students. Andrew.Ainsworth 09:40, 23 August 2007 (UTC)
The section on the reversed cycle is really a red herring. All reversed cycles are refrigeration/heat pump ones. The vapour compression cycle is really derived from the Carnot rather than the Rankine cycle. Should this section just be removed? (Donebythesecondlaw 13:28, 19 September 2007 (UTC))
i have a question,do somebody known which software can make a organic Rankine cycle T-S diagram???
Various changes.
[edit]I inflict (lecture?!) thermodynamics to undergraduates,and I have made a couple of changes to this article.
I have put in a few more diagrams for the reheat and regenerative cycles. I have a load more, but I wanted to keep the size down.
I have moved the description to the top, to let people know what they are reading about.
I have also put in a few more technical bits about overall efficiencies and Carnot efficiencies.
I have also clarified the reverse Rankine cycle bit. This is still a bit tenuous and it migh be best to remove it entirely! (Donebythesecondlaw 13:32, 19 September 2007 (UTC))
New image
[edit]Replaced layout picture with better quality one, opinions? Andrew.Ainsworth (talk) 16:48, 29 November 2007 (UTC) I've just rewritten the description section, certain statements seemed ambiguous to me ("In general terms the Rankine cycle is similar to a Carnot cycle but with the compression process taking place on a liquid") and the order of material didn't seem to flow either. Perhaps a bit long now though, some of that text may belong in another part of the article really. I thought the picture was also best updated as now people can see where the phase change occurs. Andrew.Ainsworth (talk) 01:50, 30 November 2007 (UTC)
Fixed a mistake in the equations too: where h4,s is the enthalpy after an isentropic expansion. Andrew.Ainsworth (talk) 02:00, 30 November 2007 (UTC)
Description section
[edit]What is "heat addition temperature"? --Milkbreath 16:42, 2 December 2007 (UTC)
This whole paragraph is a bit odd, and actually contradicts somewhat the next paragraph. These should be concatenated and the numbers (such as efficiencies, 42% includes boiler efficiency) reconciled. Donebythesecondlaw 16:19, 3 December 2007 (UTC)
I have tried to sort this out. Donebythesecondlaw 11:36, 4 December 2007 (UTC)
The paragraph was a bit odd on reading again. Something about the following is what I was trying to include. Perhaps the section on alternatives to the basic Rankine cycle is where it's needed. What I meant by heat addition temperature was the average temperature of heat addition defined by:
In a similar way the rejection temperature is defined by:
and the efficiency of the cycle will be given:
As the exhaust temperature is constant the only factor in increasing efficiency is increasing the inlet temperature, so superheating or feedheating would be ways to go. Andrew.Ainsworth (talk) 21:42, 7 December 2007 (UTC)
I put in the first of these equations along with a description. Can anyone shrink it a bit? Donebythesecondlaw (talk) 09:55, 21 December 2007 (UTC)
Just noticed I made a mistake above, the efficiency would be given by 1 - Tout/Tin. Andrew.Ainsworth (talk) 17:55, 22 December 2007 (UTC)
Processes of the Rankine cycle section
[edit]Last sentence: what does it mean for a Rankine cycle to be "exposed"? --Milkbreath 16:54, 2 December 2007 (UTC)
Not a clue; probably best to remove it. On a separate note, how do we get the Wikipedia credibility of this raised? It is beginning to look good. Donebythesecondlaw 16:11, 3 December 2007 (UTC)
- Don't imagine for one second that I have the first clue about thermodynamics. I don't. I just bounce around copyediting, and I like things to make sense even when I don't understand what that sense is. As for raising the profile, one can submit an article for different reviews. I believe the first step is to submit for peer review. You should probably also bring this up on the talk page for WikiProject Physics. I would do it myself, but whoever does it should be able to respond to criticisms and suggestions by altering the content, and, as I said, that ain't me. --Milkbreath 18:05, 3 December 2007 (UTC)
I have tried to sort out the "exposed" sentence. It now makes sense, but may not say the right thing. Donebythesecondlaw 11:36, 4 December 2007 (UTC)
For large central station power plants in public utility service, it is not uncommon for the boiler / turbine to operate above the critical point. This moves the turbine operation to the left on the Entropy (S) sacle, increasing the delta H (entropy) available for conversion to shaft power & thus increasing ovearll cycle efficiency. Chas in BR (talk) 20:33, 3 August 2009 (UTC) Perhaps this super-critical version could become one of the == Variations of the Rankine cycle section ==" Chas in BR (talk) 20:35, 3 August 2009 (UTC)
How about a P-v diagram?
[edit]Could come in handy for someone, you never know.
PV pdiagrams are only of interest for closed systems. As the integral of pdv is the work. — Preceding unsigned comment added by 84.51.137.53 (talk) 18:21, 9 March 2015 (UTC)
Questioning statement about lost heat of vaporization
[edit]As an engineer I once worked in a steam plant and as best I can I racall, some condensation was allowed in the turbine, up to a maximum of about 12% moisture, after which erosion of the turbine blade by the water droplets was serious. I believe the statement made at steam turbine#Steam Supply and Exhaust Conditions stating 10% exit moisture is probably correct. Phmoreno (talk) 14:07, 29 October 2010 (UTC)
Inline citations
[edit]This is not my field of expertise but I would like to check the text against citations- could someone devote a little time to providing some! A start class article:= The article has a usable amount of good content but is weak in many areas, usually in referencing. This describes this perfectly- it is not a C class article till this is rectified.--ClemRutter (talk) 12:22, 5 January 2011 (UTC)
Inconsistency
[edit]If the entry temperature of steam turbines is allegedly limited to 525 degrees because of the creep limit of stainless steel, then how do gas turbines have an entry temperature of 1500 degrees ? Why not make the steam turbines out of whatever it is that the gas turbines are made from - presumably not stainless steel. Actually I think the alleged entry temperature limit of steam turbines is spurious.Eregli bob (talk) 15:55, 2 August 2011 (UTC)
Gas turbines use different materials for blades than steam turbines. Gas turbines use Co-Cr superalloys and the blades are cast as single crystals.McNiel (1990) pps 126-8. There are technical difficulties in producing steam at ultra high temperatures: what are you going to use for boiler tubes and steam piping?Phmoreno (talk) 19:07, 2 August 2011 (UTC)
In An Encyclopedia of the History of Technology McNeil (1990), a super-critical turbine is mentioned operating with 621 C steam at the inlet, but it goes on to say that "by 1955 the average steam temperature reached its present plateau of 566 C." This is a ferretic stainless with 11-13% Cr.Phmoreno (talk) 18:56, 2 August 2011 (UTC)
Gas turbine parts exposed to hot gas are protected by various forms of air cooling. 86.181.114.173 (talk) 11:25, 14 September 2011 (UTC)
Energy loss
[edit]< This (evaporation) energy is lost to the cycle because the condensation that can take place in the turbine is limited to about 10% in order to minimize blade erosion >
This is not correct. The energy is lost to the cycle because of the second law! The shape of the saturation line (and hence the steam condition at condenser entry) has nothing to do with it. 86.181.114.173 (talk) 11:35, 14 September 2011 (UTC)
Description
[edit]'The efficiency of the Rankine cycle is limited by the high heat of vaporization of the working fluid'
Try as I might, I cannot make any sense of that statement. It may have to go.
109.145.108.66 (talk) 21:23, 13 July 2015 (UTC)
Assessment comment
[edit]The comment(s) below were originally left at Talk:Rankine cycle/Comments, and are posted here for posterity. Following several discussions in past years, these subpages are now deprecated. The comments may be irrelevant or outdated; if so, please feel free to remove this section.
| This article looks quite good now. It would be nice to get it rated. Donebythesecondlaw (talk) 19:49, 25 March 2008 (UTC) |
Last edited at 19:49, 25 March 2008 (UTC). Substituted at 03:55, 30 April 2016 (UTC)
Under the Equations for the Rankine Cycle
[edit]I feel some more detailed information should be added like this accordingly to demonstrate the stages of how the Rankine Cycle works and it would as well help when making use of the Equations in calculations on Rankine Cycle to know what equation is next for usage;
===========================
[edit]The Rankine Cycle works in this order continuously; Pump===>Boiler===>Turbine===>Condenser.
For Pump work
Note: The function of the ```Pump``` is to move fluid(liquid or gas) into the ```Boiler```, so in that process, a work is done, to calculate that work done, we have to use the equation below;
w(pump) = [dot]W(pump) / m(dot) = h2 - h1
For Boiler( heat addition);
Note: The function of the ```Boiler``` is to increase the temperature of the fluid(liquid or gas) moved into it(the Boiler) by the Pump, so in that process, there is an added heat, to calculate the heat added, we have to use the equation below;
q(in) = [dot]Q(in) / [dot]m = h3 - h2
For Turbine work; Note: A Turbine is a rotary machine that uses the kinetic energy of a continuous stream of fluid(liquid or gas) to turn a shaft. Therefore, a turbine does a work, to find the work done by the turbine, we have to use the equation below
w(turb) = [dot]W(turb) / m(dot) = h3 - h4
For Condenser( heat rejection);
Note: The function of the ```Condenser``` is to cool the temperature of the fluid extracted into it(the Condenser) by the ```Turbine``` , so in that process, there is an added heat, to calculate the heat added, we have to use the equation below;
q(out) = [dot]Q(out) / [dot]m = h4 - h1
Note: the fluid(liquid or gas) extracted into the condenser from the turbine is then moved by the pump again [i.e it returns to the beginning and then repeats itself in the same sequence(Cycle)].
===============================
[edit]Thank you. I would have loved to edit it myself, but I'm having difficulties editing the mathematical expressions, so i dont want to mess with. Its saying parse error. Victoriod (talk) 11:55, 17 July 2019 (UTC)





